
Considerations For Implementing Embryo Transfer
Evolution of Embryo Transfer

Nearly 132 years ago, in 1891, embryo transfer (ET) was achieved using rabbits. However, it took an additional 80 years for embryo transfer to be adopted as a routine practice within the cattle industry. Over the last half century, embryo transfer has revolutionized cattle reproduction. This technology allows genetically superior females to have multiple calves per year through a surrogacy process in recipient females. Where producers used to be limited to one high dollar calf per year from their best female, now they can collect oocytes from their best female, fertilize them using in-vitro fertilization, and transfer all viable embryos into recipients. Thus, embryo transfer is a technique to quickly multiply the genetics of top females in a herd.
Embryo transfer has also been proven to have improved success rates compared to artificial insemination (AI) during heat stress (Hansen et al., 2019). Embryos succumb to heat stress prior to day 7 of gestation, but in-vitro fertilized embryos transferred at the blastocyst phase have acquired resistance to effects of maternal heat stress (Hansen, 2020). Thus, utilizing ET during summer months or in the southeastern United States, especially in dairy operations, can have positive impacts on reproductive success. Moreover, ET was proven to be superior to AI in repeat breeder dairy cows. These are cows that fail to conceive from 2 or more insemination services in the absence of reproductive abnormalities. A study by Rodrigues et al. (2007), found that ET cows had a 10% increase in pregnancies compared to AI. The reason for this improvement is unknown, but researchers speculate that oocyte quality and early uterine function could be negatively impacted in repeat breeders (Sood et al., 2017; Katagiri and Takahashi, 2004). In ET, neither of these variables contribute to the survivability of the transferred embryo.
Embryo transfer pregnancy rates can be upwards of 65% starting at transfer to the delivery of a live calf. It is a slightly more expensive technology but can create a valuable calf crop that can bring in a higher profit. When deciding to implement embryo transfer in your operation, there are several factors to consider:
- Type of Embryo
- Frozen vs. Fresh
- In-vitro vs. In-vivo produced
- Embryo Grading
- Best Method of Embryo Transfer Use
- Labor
- Facilities
Utilizing Fresh Versus Cryopreserved Embryos
The first factor when implementing ET is deciding whether to purchase cryopreserved embryos or to flush embryos from donors within the herd for fresh embryo transfer. The simplest way to answer this question is to decide if one wants to use their own herd genetics or purchase embryos created from an external dam and sire. The next question is if one wants to use embryos created through in-vitro fertilization or in-vivo (fresh flushed) embryos. There are pros and cons to each embryo type, but ultimately the choice is dependent on producer goals.
Cryopreserved Embryos
Cryopreserved embryos that are frozen prior to embryo transfer. This allows for shipping and later transfer dates. Thus, genetically superior cryopreserved embryos are readily available for purchase. The calves produced from these pregnancies are more valuable at weaning. These calves can also be used to increase genetic diversity within a herd. Embryos flushed from herd donors can also be frozen to be used at a later date. Although a lot of research has been dedicated to improving cryopreservation, there is still lower success rates when using frozen embryos compared to thawed (Numabe et al., 2000). We recommend using cryopreserved embryos when seeking external genetics to improve diversity and create a more profitable calf crop.
- Pros:
- Readily available for purchase
- Only need recipient cows (no need for donors)
- Increase value of calves produced
- Increase genetic diversity
- Cons:
- Lower pregnancy rates compared to fresh embryos
- More expensive to purchase
Fresh Embryos
Fresh embryos are not frozen, meaning these embryos are either flushed directly from donors or the in-vitro culture media and transferred into recipient cows. While fresh embryos have higher success rates, they are often more difficult to obtain and transfer than frozen embryos. Using fresh embryos requires local use of either in-vitro techniques or setting up and breeding donor cows to flush. This is recommended if the producer would like to transfer embryos from cows within the herd. Using fresh embryos will increase success rates (Ferraz et al., 2016).
- Pros:
- Increased success rates
- Use of herd genetics
- Cons:
- Need to use advanced techniques (in-vitro fertilization and ovum pick-up)
- More in-depth protocols
- Can be expensive
In-vitro Produced Embryos
The ability to produce embryos from in-vitro fertilization has been a huge advancement in embryo transfer. However, due to the inability to exactly replicate the uterine environment, these embryos often have lower success rates than in-vivo produced (flushed) embryos. The use of in-vitro fertilization to created frozen embryos that can be shipped decreases animal handling work. We recommend using in-vitro embryos if one wishes to limit donor handling, freeze embryos for later dates, or produce a high number of embryos.
- Pros:
- Limit donor handling
- Can be frozen for later use
- Can produce a high number of embryos
- Can use cows that have issues maintaining pregnancy
- Cons:
- Culture media is not as effective embryo environment as the uterus
- Lower success rates
- Laboratory equipment required
- Ovum pick-up skill required
In-vivo Produced (Fresh) Embryos
Fresh embryos are created by super ovulating donor cows, breeding donor cows, and flushing embryos from the uterus at day 6-8 of gestation. These embryos can either be transferred immediately or frozen for use later. Fresh embryos have higher success rates than in-vitro produced embryos. However, if one does not own donor cows or donor cows have issues maintaining pregnancy, this route could be expensive with only 1-2 embryos flushed at day 6-8. Moreover, if doing fresh flushed embryo transfer directly into recipient cows, producers must have clean and durable facilities that can handle many cattle.
- Pros:
- High success rates with fresh embryos
- Use of herd genetics
- More uniform calf crop
- Cons:
- Labor requirements
- Must synch donors and recipients to do fresh transfer
- Requires clean and durable working facilities
Embryo Grading
Regardless of what embryo type is used (fresh vs. frozen; in-vitro vs. in-vivo) all embryos undergo a screening process to ensure there is a high likelihood the embryo can result in a pregnancy. This process is referred to as embryo grading. The scoring system was developed and recommended by the International Embryo Transfer Society.
While widely used today, embryo grading is still a subjective analysis. Grading is performed by evaluating fresh embryos under a stereomicroscope to determine quality prior to cryopreservation or embryo transfer. Embryos are assigned a two-digit code consisting of the stage (1-9) and quality code (1-4) based off the microscopic visual evaluation.
Stage Code
The stage code is based off the stage of development and ranges from 1-9. For embryo transfer, it is only recommended to use embryos that are in stages that are normal on embryo recover at day 7. In super ovulated cows, one can observe a wide range of embryo stages upon flushing. It is important to identify the correct stage and determine if the embryo can be utilized. The highest pregnancy rates are from using stage 4-7 embryos. These are the stage types recommended to use for cryopreservation purposes as well. After embryos receive their stage code, they are then evaluated for quality.
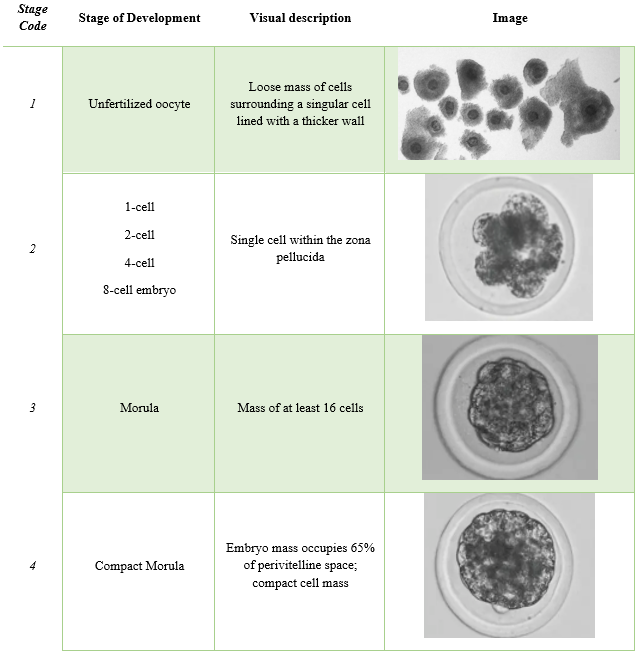
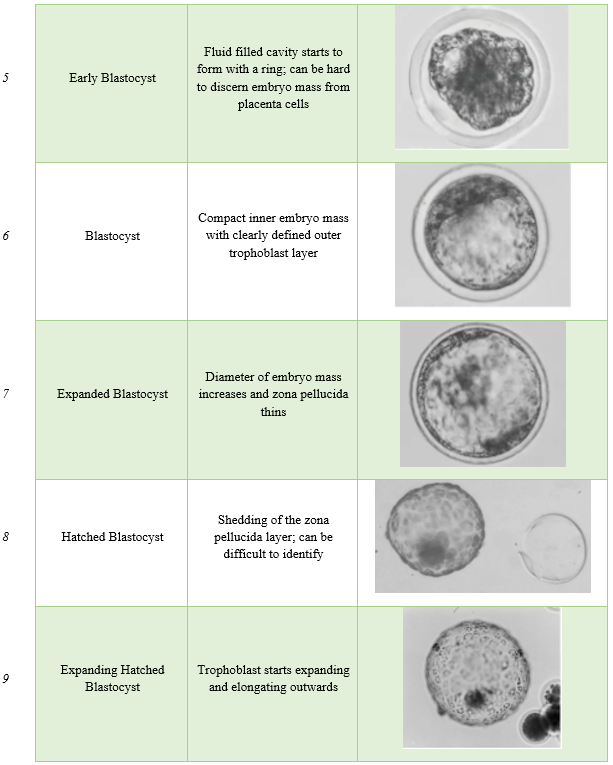 Images were taken by Dr. Brad Lindsey; adapted from (Janke et al., 2017).
Images were taken by Dr. Brad Lindsey; adapted from (Janke et al., 2017).Embryo Grade
The second number given to an embryo is the quality code. This is from a scale of 1-4. The quality of the embryo is a subjective measure based on the experience of the technician. However, they focus on the several key features to determine quality.
- Spherical shape
- Symmetrical
- Uniform blastomeres (inner cells)
- Size
- Shape
- Color
- Density
In the table below, one can observe the differences in shape, symmetry, and uniformity of the inner cells as the quality decreases.
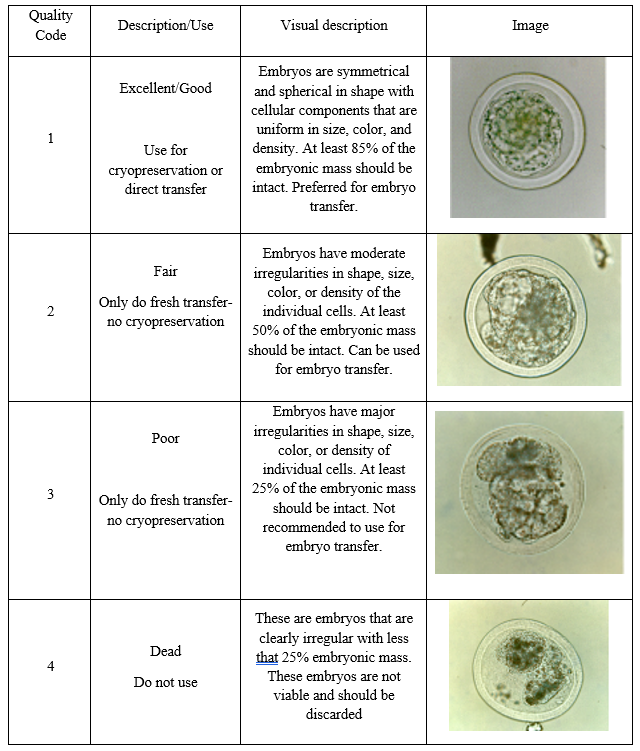 Images adapted from (Bo & Mapletoft, 2013)
Images adapted from (Bo & Mapletoft, 2013)Strategies To Implement Embryo Transfer
Embryo transfer protocols will place a lot of pressure on one’s facilities. These protocols require different number of working times depending on producer goals. Thus, when choosing what types of embryos to use, one should be aware of the labor and facility requirements. At the minimum, producers should have a working chute with a palpation gate, sturdy alleyway, and tub for moving cows into the chute. The quality of these facilities will determine the quantity of animals that can be worked at a given time.
Using Cryopreserved Embryos:
To use cryopreserved embryos, all recipients need to undergo an estrus synchronization protocol. The recommended protocols for ET normally have 3 working timepoints. Seven days after the AI date, embryos will be transferred into recipients. (Note: recipients are not bred on day of AI in the protocol, this is just a reference for when the recipient uterus will be best for embryo transfer). Cows on this protocol will have 4 working timepoints.
If choosing this option, labor and facility requirements are dependent on the number of recipient animals used. We recommend to only synchronize a set number of cows that can be easily worked through your facility within 3-4 hours.
Super ovulation In-Vitro Ovum pick-up:
In this protocol, donor cows will need to be given a shot twice daily for 3-4 days and ovum pick-up will be done on day 5. Thus, all donors will need to be worked a minimum of 7 times over the course of 5 days.
It is easiest to keep donors close to the working facility when giving the series of superovulation injections. Thus, producers should limit donor numbers dependent on pen and pasture availability close to the working facility.
Depending on the technician, ovum pick-up may need to be done at a facility with a conjoined laboratory space. If this is the case, consider the transportation costs and increased labor that will be associated with this protocol.
Super ovulation In-Vivo Flushing for Cryopreservation:
In this protocol, donor cows will need to be given a shot twice daily for 3-4 days and then artificially inseminated on day 5 and day 6. Then, 6-8 days after AI, embryos will need to be flushed from the dam and collected to undergo a cryopreservation protocol.
In addition to working times, this protocol will require a clean area close to the chute to recover embryos under the microscope and start the cryopreservation process. This can be a difficult protocol to implement on farm and often ET clinicians request cows be brought to their facility for flushing and cryopreservation. If this is the case, consider transportation costs and increased labor associated with this protocol.
Super ovulation In-Vivo Flushing & Fresh Transfer:
This protocol has the highest success rates, but also has the highest demand on working facilities and highest labor costs.
In this protocol, donor cows will need to be given a shot twice daily for 3-4 days and then artificially inseminated on day 5 and day 6. Then, 6-8 days after AI, embryos will need to be flushed from the dam and collected to be immediately transferred into recipients.
Recipient cows will need to undergo an estrus synchronization protocol. The recommended protocols for ET normally have 3 working timepoints. The estrus synchronization AI timepoint must align with day 5 of the donor protocol. Then when embryos are flushed 6-8 days later, they can be transferred into a similar uterine environment.
This protocol will require all cows being used to be worked through the facilities on the same days. Thus, depending on the ease of moving animals through and the skill of technicians, this could lead to very intense labor days.
Summary
Deciding to use embryo transfer at one’s facility is an effective way to increase calf value and introduce new genetics into a herd. However, producers must understand the pros and cons that come with using certain embryo types and embryo transfer protocols. Producer decisions should be dependent on the quality of their facilities, labor, and operation goals. In general, protocols that have less working timepoints will be easier to implement. Courses offered at International Embryo Technology School discuss these options further to help producers decide the best protocol for their operation and realize the full economic benefit of utilizing embryo transfer at their operation.
References:
Bo., G.A., & R.J. Mapletoft. 2013. Evaluation and classification of bovine embryos. Animal Reproduction. 10(3): 344-8
Ferraz, P.A., C. Burnley, J. Karanja, A. Viera-Neto, J.E. Santos, R.C. Chebel, and K.N. Galvao. 2016. Factors affecting the success of a large embryo transfer program in Holstein cattle in a commercial herd in the southeast region of the United States. Theriogenology. 86: 1834-41
Hansen, P.J. 2019. Reproductive physiology of the heat-stressed dairy cow: implications for fertility and assisted reproduction. Anim. Reprod. 16: 497-507
Hansen, P.J. 2020. The incompletely fulfilled promise of embryo transfer in cattle- why aren’t pregnancy rates greater and what can we do about it? Journal of Animal Science 98(11): 1-20
Janke, M.M., J.K. West, & C.R. Youngs. 2021. Evaluation of In Vivo-derived bovine embryos. Ch. 79: In Bovine Reproduction 2nd Edition. Editor: Richard M. Hopper. Wiley Publishing.
Katagiri, S., and Y. Takahashi. 2004. Changes in EGF concentrations during estrous cycle in bovine endometrium and their alterations in repeat breeder cows. Theriogenology. 62: 103-12
Namabe, T., T. Oikawa, T. Kikuchi, and T. Horiuchi. 2000. Birth weight and birth rate of heavy calves conceived by transfer of in vitro or in vivo produced bovine embryos. Anim Reprod Sci. 64: 13-20
Rodrigues, C.A., H. Ayres, R.M. Ferreira, A.A. Teixeria, R.F. Mancilha, M.E.F. Oliveira, A.H. Souza, and P.S. Baruselli. 2007. Comparison of pregnancy rates after artificial insemination or embryo transfer in high producing repeat breeder Holstein cows. Acta Sci Vet. 33(Suppl. 1): 1255
Sood, P.M., M. Zachut, I. Dekel, H. Dube, S. Jacoby, and U. Moallem. 2017. Preovulatory follicle characteristics and oocyte competence in repeat breeder dairy cows. J Dairy Sci. 100: 9372-81

|
|
|